The rise of Artificial Intelligence (AI) in healthcare is transforming the medical device landscape at an unprecedented pace. A closer look at the latest FDA approvals of devices enabled by AI and machine learning (ML), released in August 2024, reveals significant trends – from a surge in approvals to AI’s expanding role in fields like radiology, neurology, and gastroenterology.
1. Approval Growth Over Time
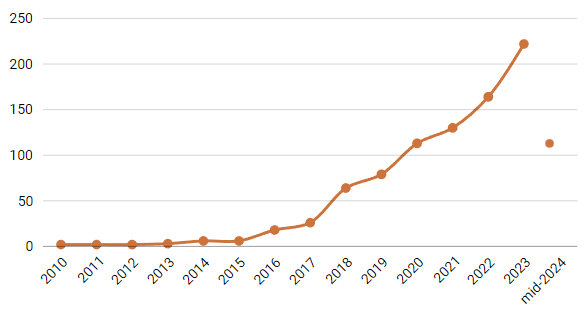
While the FDA has tracked AI/ML-enabled devices since 1995, only 11 approvals were granted between 1995 and 2009. From 2010 to 2015, approvals remained modest, averaging just two or three per year. However, a sharp upward trend began in 2016, accelerating AI’s entry into healthcare.
The numbers tell the story: by 2023, a record 221 devices had been approved, a dramatic leap from just six approvals in 2015. By mid-2024, another 107 devices had already received FDA clearance—an impressive figure considering it reflects only the first six months of the year.
2. Top Medical Fields for AI/ML Adoption
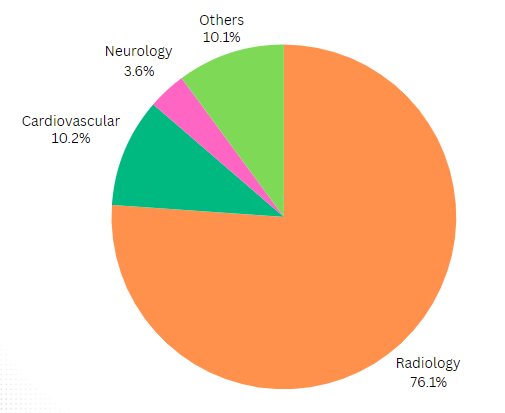
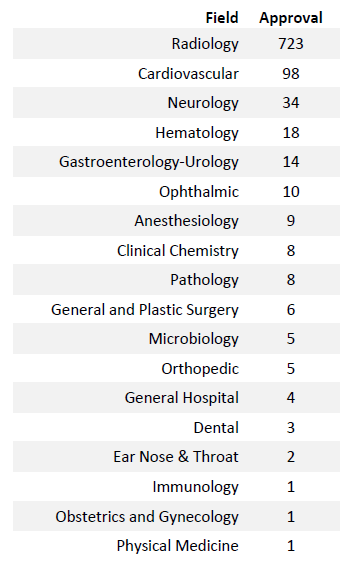
Radiology is leading the AI revolution, with 723 approved devices to date. Other specialties, such as cardiovascular care (98 approvals), neurology (34), and hematology (18), are also increasingly adopting AI and machine learning to enhance diagnostic precision and streamline data analysis.
3. Leading Companies in AI/ML Medical Device Approvals
Several companies have emerged as leaders in AI/ML-enabled device approvals between 2010 and 2024. Siemens Medical Solutions USA, Inc. stands out with 47 FDA-approved devices, making them a key player in the AI healthcare space. Canon Medical Systems Corporation (28 approvals) and Aidoc Medical, Ltd. (24 approvals) also hold significant shares in AI-driven medical innovation.
International competitors are gaining ground as well. During the same period (2010–2024), companies like Shanghai United Imaging Healthcare in China (17 approvals) and Zebra Medical Vision in Israel (9 approvals) have also made notable progress. Their presence underscores the growing global competition and the increasingly international nature of AI development in healthcare.
4. Emerging Fields and Significant Growth Areas
Beyond established fields like radiology and cardiology, several emerging areas are experiencing rapid growth in AI adoption:
-
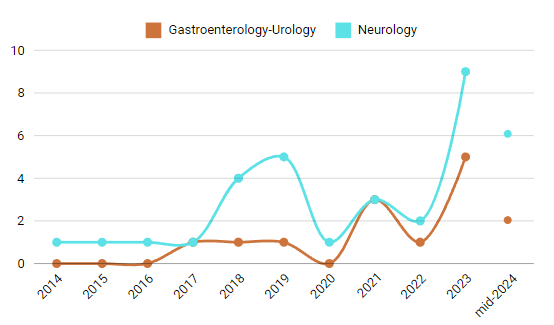
Neurology: Approvals for AI/ML-enabled devices in neurology have surged in recent years. Prior to 2014, no AI devices had been approved in this field. Approvals gradually began with one per year from 2014 to 2017, but from 2018 onwards, the numbers climbed rapidly, reaching nine approvals in 2023 and six more by mid-2024.
-
Gastroenterology and Urology: These specialties saw no AI device approvals before 2014, with only one approval annually between 2017 and 2019. By 2023, approvals had risen to five, with two more by mid-2024. This growth highlights AI’s role in enhancing diagnostic accuracy and procedural efficiency in these fields.
As a U.S.-based company with 30 years of experience, Source Machining Specialties specializes in helping U.S. manufacturers with their manufacturing needs in India. We’re so confident in our Indian production facilities that we invite you to a site audit at our expense. Discover more about our capabilities and services, and let’s start a conversation.